Danvers, Massachusetts based Abiomed, Inc. has received U.S. Food and Drug Administration (FDA) approval under a Humanitarian Device Exemption (HDE) for its Impella RP (Right Percutaneous) single access heart pump System. The Impella RP is the first percutaneous medical device designed for right heart support to receive FDA approval. Abiomed completed the HDE submission for the Impella RP in September 2014 upon completion of the RECOVER RIGHT clinical study.
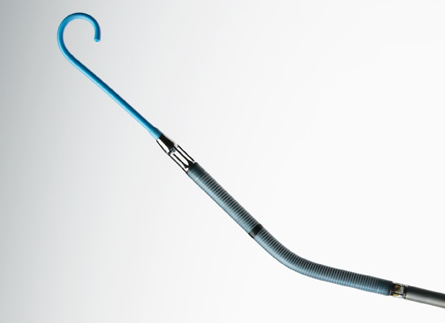
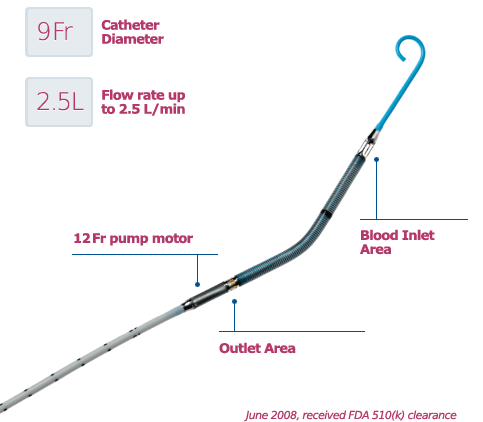
The Impella RP Catheter delivers blood from the inlet area, which sits in the inferior vena cava, through the cannula to the outlet opening near the tip of the catheter in the pulmonary artery. The Impella RP is FDA indicated for delivery through a standard catheterization procedure via the femoral vein, into the right atrium, across the tricuspid and pulmonic valves, and into the pulmonary artery. The catheter insertion requires only a small incision in the femoral vein of pediatric or adult patients with a body surface area 1.5 m2 who develop acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery, in order to provide circulatory assistance. The Impella RP is implanted in a hospital, and patients receiving the device must stay in hospital until it is removed — for up to 14 days.
In patients with a deficiency of blood flow from the right side of the heart, the Impella RP is designed to restore the flow and pressure required to compensate for right heart failure and to help reduce the amount of work a patient’s own heart must do. It assists in pumping blood from outside the heart, from the inferior vena cava, through the heart and into the pulmonary artery. Once implanted, the Impella RP Catheter delivers blood from the inlet area, which sits in the inferior vena cava, through the cannula to the outlet opening near the tip of the catheter in the pulmonary artery.
While the Impella RP is working, the patient’s heart has time to rest and recover its ability to pump blood. Once the patient’s right heart is working on its own again, the Impella RP can be removed. If the patient’s right heart does not recover within two weeks, the patient may need a different treatment such as a permanent heart pump or a heart transplant. In the U.S. clinical trial, about 3 out of 4 patients who received the device survived to at least 30 days after the device was removed.
Patients who may use the device include:
• Patients whose right heart fails after receiving a heart pump to support the left side of the heart.
• Patients whose right heart fails because of a heart attack.
• Patients whose right heart fails after a heart transplant.
• Patients whose right heart fails after heart surgery.
Counterindications include physical conditions that may interfere with the placement or performance of the device such as defects in the patient’s veins and arteries, including calcium deposits or hardening of the blood vessel walls or a replacement heart valve, a blood filter in one of the patient’s large veins, or severe narrowing of one of the patient’s heart valves — all of which could block clearance available for the pump to pass.
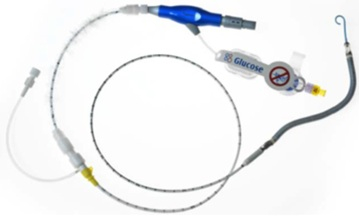 The Impella RP System consists of a mini heart pump mounted at the end of a thin, flexible tube (catheter), a console that drives the pump, and an infusion pump that flushes the pump. The heart pump can be implanted in the right side of the heart to help pump blood in patients who need short-term support. The Impella RP device, which doesn’t require open chest surgery surgery for insertion, can provide hemodynamic support of up to four liters of bood flow per minute.
The Impella RP System consists of a mini heart pump mounted at the end of a thin, flexible tube (catheter), a console that drives the pump, and an infusion pump that flushes the pump. The heart pump can be implanted in the right side of the heart to help pump blood in patients who need short-term support. The Impella RP device, which doesn’t require open chest surgery surgery for insertion, can provide hemodynamic support of up to four liters of bood flow per minute.
 “The Impella RP represents a huge step forward in offering right side support using a minimally invasive platform and has the potential to transform interventional cardiology and cardiac surgery today. With the ability to place this device percutaneously on the right side, physicians can now treat acute right sided heart failure minimally invasively and quickly,” says Mark Anderson, M.D., co-principal investigator for the RECOVER RIGHT trial and chair of the division of cardiothoracic surgery at Einstein Medical Center in Philadelphia, Pennsylvania, who adds that “The trial results were favorable and the 30-day outcomes appear promising compared with clinical data from surgical RV assist devices; however our study was not designed for a statistical comparison. Overall, the results show that the percutaneous approach with Impella RP potentially offers significant advantages to patients.”
“The Impella RP represents a huge step forward in offering right side support using a minimally invasive platform and has the potential to transform interventional cardiology and cardiac surgery today. With the ability to place this device percutaneously on the right side, physicians can now treat acute right sided heart failure minimally invasively and quickly,” says Mark Anderson, M.D., co-principal investigator for the RECOVER RIGHT trial and chair of the division of cardiothoracic surgery at Einstein Medical Center in Philadelphia, Pennsylvania, who adds that “The trial results were favorable and the 30-day outcomes appear promising compared with clinical data from surgical RV assist devices; however our study was not designed for a statistical comparison. Overall, the results show that the percutaneous approach with Impella RP potentially offers significant advantages to patients.”
“We are pleased to receive the HDE approval for the Impella RP. This is a milestone for the company as well as the interventional community in providing support for patients with right sided heart failure. Our Impella platform now has the ability to offer support for both sides of the heart, offering more treatment options for patients and hospitals,” comments Michael R. Minogue, Chairman, President and Chief Executive Officer, Abiomed.
There will be a controlled launch of the Impella RP in the U.S. after each site completes a rigorous in-house training program at Abiomed incorporating members of the heart team, including the interventional cardiologist, cardiac surgeon, heart failure cardiologist and lead nurse. Abiomed also provides online training resources for the Impella RP device here:
http://www.abiomedtraining.com/
As part of the HDE approval, Abiomed is required to conduct two post approval studies (PAS) for the RECOVER RIGHT trial. One includes an adult patient population of 30 patients and the other, a pediatric patient population for a maximum of 15 patients (larger patients < 18 years of age with RVF.) These studies will be conducted to monitor the post-market safety and probable benefit of the Impella RP device. Both studies will be a single-arm multi-center studies that will follow the respective patients at 30 and 180 days post device explant.
RECOVER RIGHT Study Results
RECOVER RIGHT was an FDA-approved, prospective, multi-center, single arm study designed to establish that the use of Impella RP is safe, feasible and provides a hemodynamic benefit in patients with right ventricular failure refractory to medical treatment and deemed to require hemodynamic support.
The 30 patients enrolled in the trial were divided into two patient cohorts; Cohort A including patients who developed RVF within 48 hours after implantation of a left ventricular assist device (LVAD), while Cohort B investigated patients who developed RVF within 48 hours of post-cardiotomy shock or post-acute myocardial infarction (AMI) shock. Primary endpoints were patient survival at 30 days, hospital discharge, or bridge to subsequent additional therapy.
Overall, survival rate among the enrolled subjects was 73 percent in the entire population at 30 days. Cohort A showed a survival rate of 83.3 percent and Cohort B showed a 58.3 percent survival rate at 30 days.
 “Right-side heart failure carries a high risk of mortality, and historically has been difficult for physicians to treat minimally invasively. The data from this trial is encouraging, and demonstrates that the Impella RP may play a pivotal role in the treatment of RVF patients in need of hemodynamic support in the future here in the U.S.,” says William O’Neill, M.D., co-principal investigator for the RECOVER RIGHT trial and medical director of structural heart disease at http://www.henryford.com/body.cfm?id=59142&maptype=h Henry Ford Hospital system throughout greater Detroit, MIchigan.
“Right-side heart failure carries a high risk of mortality, and historically has been difficult for physicians to treat minimally invasively. The data from this trial is encouraging, and demonstrates that the Impella RP may play a pivotal role in the treatment of RVF patients in need of hemodynamic support in the future here in the U.S.,” says William O’Neill, M.D., co-principal investigator for the RECOVER RIGHT trial and medical director of structural heart disease at http://www.henryford.com/body.cfm?id=59142&maptype=h Henry Ford Hospital system throughout greater Detroit, MIchigan.
The RECOVER RIGHT clinical trial results “A Prospective, Multicenter Study to Evaluate a New Percutaneous Ventricular Assist Device for Right Ventricular Failure: The RECOVER Right Study,” were presented by Dr. O’Neill at the annual Transcatheter Cardiovascular Therapeutics (TCT) 2014 scientific meeting in Washington, DC in October 2014.
The FDA approval letter for the Impella RP System can be found at:
http://www.accessdata.fda.gov/cdrh_docs/pdf14/H140001a.pdf
Sources:
Abiomed, Inc.
clinicaltrials.gov
Image Credits:
Abiomed, Inc.
Einstein Medical Center
Henry Ford Hospital