 A team led by researchers from the University of Vermont’s Cardiovascular Research Institute recently reported findings on a muscle protein and its central role in proper heart functioning. The study was published in the journal Science Advances and is entitled “Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling.”
A team led by researchers from the University of Vermont’s Cardiovascular Research Institute recently reported findings on a muscle protein and its central role in proper heart functioning. The study was published in the journal Science Advances and is entitled “Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling.”
The human heart is considered a fine-tuned engine. Researchers have now found that a specific element of this engine plays a central role in the maintenance of the heart’s precise tuning — this element is known as myosin-binding protein C (cMyBP-C).
The heart fails when the cMyBP-C protein is not working properly, as is the case in hypertrophic cardiomyopathy, a condition frequently asymptomatic until sudden cardiac death, where a part of the myocardium (the heart muscle) becomes abnormally thick (hypertrophied) without a clear cause. This condition is the leading cause of “sudden death” in healthy young athletes.
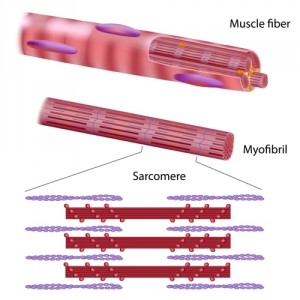
Powerful microscopes were used to study the heart sarcomere — the basic contractile unit of the muscle that is composed of long, fibrous proteins that slide past each other as the muscles contract or relax with every heartbeat. Trillions or more sarcomeres must all contract simultaneously. “To pump blood efficiently, they all have to be doing it at the same time,” explained the study’s senior author Dr. David Warshaw in a news release.
There are two types of fibrous proteins in sarcomeres that help them contract, thick filaments called myosin and thin filaments called actin. Myosin acts as a motor that pushes or pulls actin. cMyBP-C is located at the middle of the sarcomere and modulates myosin by either speeding up or slowing down its interaction with actin.
Calcium plays an essential role in the sarcomere; it is released from the ends of the sarcomere at each beat and must diffuse towards the center of the sarcomere where it displaces a protein that blocks the interaction between the filaments, so that myosin can bind to actin when necessary. The team found that cMyBP-C is strategically only present in the middle of the sarcomere tube to assist calcium in its role. The location and calcium assistance of cMyBP-C is crucial for proper heart functioning.
“Our curiosity into the function of the protein [cMyBP-C] was originally piqued because we knew that issues with this protein are involved with diverse forms of cardiac disease,” said the study’s lead author Dr. Michael Previs.
“Myosin-binding protein C is a perfect modulator,” added Dr. Previs. “You can fine-tune how the engine is performing by simply manipulating the functional impact of this critically important protein.” The next step is to understand cMyBP-C malfunction in a faulty heart, and possibly develop therapeutic strategies based on cMyBP-C adjustments to treat diseased hearts.


