
Medical device company Procyrion, Inc. has invited cardiologist Manesh Patel from Duke University Medical Center to join its scientific advisory board. The physician was chosen due to his expertise in the field and will assist the company’s development process of the first catheter-deployed circulatory device to help in the ambulatory treatment of heart failure.
Patel is currently the director of Interventional Cardiology and Cardiac Catheterization Labs at the Duke University Health System, and his research is focused on improving diagnosis and intervention for coronary angiography, cardiac devices, peripheral angiography, and endovascular intervention.
“Dr. Patel’s expertise in the cath-lab and his focus on patient-centered efforts to improve cardiac catheterization procedures align nicely with what we’re working to achieve with Aortix,” said the president and CEO of Procyrion, Benjamin A. Hertzog, in a press release. “His knowledge, guidance, and capability will be great benefits as we approach our first-in-human clinical studies later this year.”
Procyrion is currently studying Aortix, which is an intra-aortic pump that enables the continuous flow of blood to the vital organs and is expected to become a minimally invasive therapeutic option for patients with chronic heart failure, but are too sick to be treated only with medication. The device is smaller than 6mm and is delivered through a catheter during an outpatient procedure that lasts about ten minutes.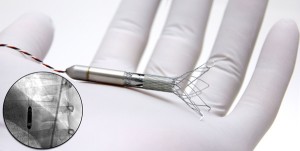
“The cardiology community is desperate for novel technologies to treat chronic heart failure,” explained Patel. “Procyrion’s innovative Aortix device has the potential to provide a significant clinical benefit to a large population of NYHA Class III-IVa patients that have few treatment options. It is a pleasure to be working with such an innovative company and team.”
The procedure includes the placement of a catheter in the femoral artery, which is held with novel self-expanding anchors. After the deployment, the catheter is fully removed, while the anchored pump and a small diameter, flexible electrical power wire remain, and may be tunneled to a transdermal exit site or to a Transcutaneous Energy Transfer system for subcutaneous implantation.
Not only is the device expected to help the 1.6 million people in the U.S. who suffer from the condition and have few therapeutic options, but it has also been awarded a $50,000 grant from the National Capital Consortium for Pediatric Device Innovation to enable the study of its pediatric use. The grant is included in its FDA Pediatric Device Consortia grant program and gave Procyrion the opportunity to collaborate with Maxon Precision Motors, and modify the adult Aortix device to be used in children born with single ventricle heart defects.
In addition to being a recent member of Procyrion’s scientific advisory board and involved in studies on both cardiovascular disease and cardiac imaging, Patel is the chairman of the committees of both the American College of Cardiology and the American Heart Association. He is also a distinguished cardiologist who has been awarded for his work with a novel integrative approach to healthcare focused on patients.
On the board Patel will be working in collaboration with the professor in the department of Internal Medicine and medical director of the Heart Failure Program at the University of Michigan, Keith Aaronson, and the medical director of the Jewish Hospital’s Heart Failure, Transplantation and Mechanical Circulatory Support Program, Emma Birks, who is also a professor of Cardiovascular Medicine with the University of Louisville.
Joseph Rogers, who serves as medical director of the Cardiac Transplant and Mechanical Circulatory Support Program and Senior Vice Chief for Clinical Affairs at the Division of Cardiology of the Duke University, and Hani N. Sabbah, a tenured professor of medicine at Wayne State University in Detroit and the Director of Cardiovascular Research at the Henry Ford Health System in Detroit, are the other members of the board.


