NeuroVive Pharmaceutical AB, a mitochondrial medicine firm, has indicated that it will announce the topline results of the company’s phase III CIRCUS study of CicloMulsion® in those suffering with ST-segment elevation acute myocardial infarction (STEMI), a type of heart attack, in the third quarter of the year.
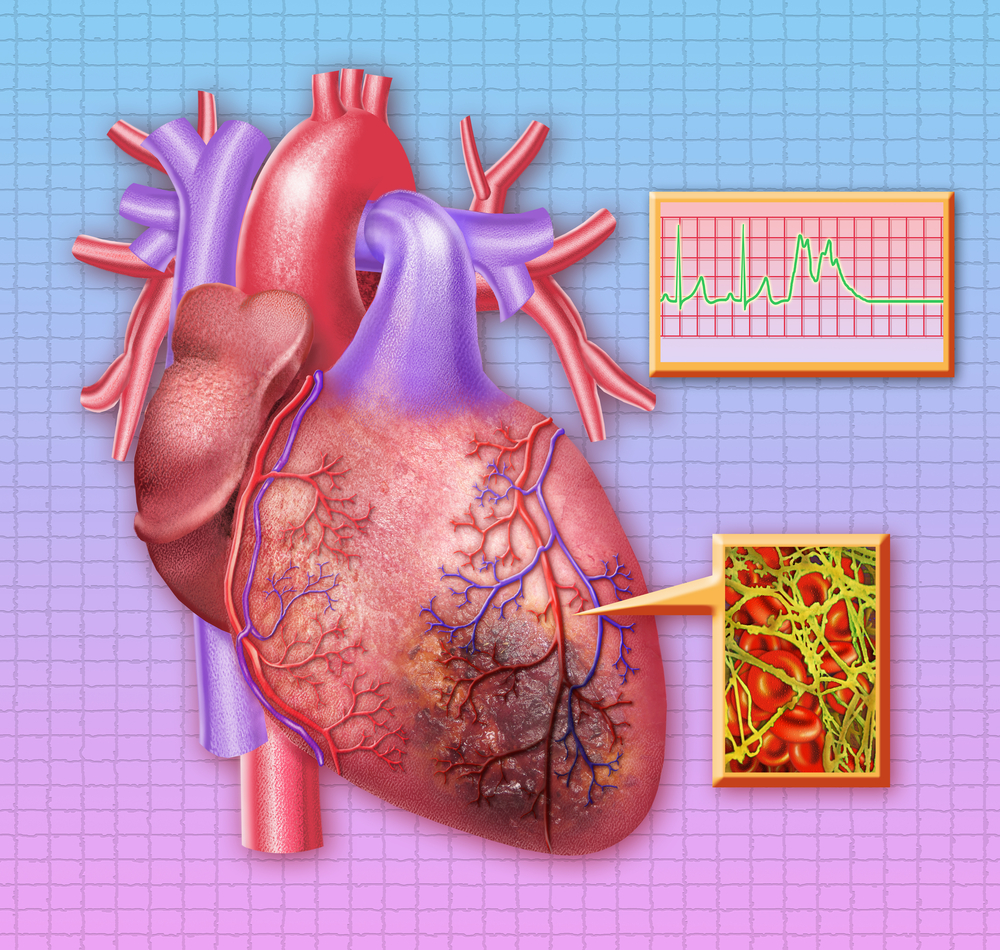
Myocardial infarction (MI) is the medical term for the more commonly known “heart attack.” MI occurs as a result of blood flow ceasing to flow in a section of the heart, which in turn causes heart muscle damage. Common symptoms associated with MI include chest pain and discomfort as well as shortness of breath, nausea, feeling faint, a cold sweat, or feeling tired.
The topline outcomes will allow company researchers to understand whether the primary endpoint of the CIRCUS study was achieved or not. The primary endpoint for the study is a composite of three different outcomes: mortality, left-ventricular remodeling and hospitalizations for heart failure. NeuorVive notes, however, that specific data about levels of significance of CicloMulsion’s efficacy will not be included in the topline results; more detailed information about the study will be revealed in a subsequent analysis and released in a final report.
In addition to the topline results, the future impact of the results concerning the advancement of CicloMulsion® will be published in the second half of 2015.
NeuroVive’s experimental therapy CicloMulsion is a lipid emulsion of cyclosporine and the first cyclophilin inhibitor in development to address reperfusion injury. The goal of developing the therapy is to prevent mitochondrial death in damaged cells and to limit the several biochemical processes that cause secondary tissue damage right after a heart attack. The company believes that CicloMulsion might protect cells and ensure that the normal regenerative mechanisms of the damaged cells are able to continue the repair process and keep the cell functional. CicloMulsion’s capacity to treat myocardial infarction is now being evaluated in a phase II study to prevent renal injury during heart surgery as well.
Read More Related Studies
NeuroVive Pharmaceutical AB recently announced the enrollment of its first patient in a clinical phase II trial that will evaluate the potential of CicloMulsion® (ciclosporin) to reduce the risk and degree of acute kidney injury after cardiac surgery. To enable surgical procedures like heart valve repair or implantation of coronary artery bypass grafts, the heart is frequently connected to a heart-lung machine during cardiac surgery. This heart-lung machine oxygenates and pumps blood throughout the body while the heart is disconnected, forming an altered blood flow and distressing the whole body. Acute kidney injury (AKI) is a common complication after cardiac surgery and is associated with decreased long-term survival. The mechanism behind this condition is unknown but ischaemia-reperfusion injury is thought to play an important role.