Pacemakers are surgically implanted medical devices that have improved and lengthened the lives of millions of cardiac patients by generating electrical impulses to treat irregular or stalled heartbeats. The U.S. Food and Drug Administration (FDA) has just approved the first pacemaker that eliminates the need for wired leads to maintain an electrical connection between the pacemaker’s pulse-generating device and the heart.
While the Micra Transcatheter Pacing System (TPS) from Mounds View, Minnesota-based Medtronic Inc. works similarly to other pacemaker products that help regulate heart rate, the self-contained, inch-long Micra TPS device is a single-chamber pacing system that paces only the right ventricle of the heart, and is implanted directly into the heart’s right ventricle chamber.
Micra is 93 percent smaller than traditional pacemakers, and about the size of a large vitamin capsule. However, the tiny device incorporates a battery that lasts as long as the batteries used in traditional pacemakers that may be up to 10 times larger in size, delivering an estimated average 12-year battery longevity.
The FDA notes that nearly one million people worldwide are implanted with pacemakers each year. In traditional single chamber pacemakers, the wire connector leads run from the pacemaker, which is implanted under the skin near the collarbone, and are routed through a vein directly into the heart’s right ventricle. These leads deliver electric pulses from the generator to the right ventricle and thereby help regulate and coordinate the chamber’s  contraction timing. Medtronics’ Micra pacemaker device eliminates these wire leads, which can sometimes malfunction or cause infections in surrounding tissues, necessitating a surgical procedure to replace the device. Due to Micra’s miniature size and minimally invasive installation approach, it leaves no lump or other visible sign of a medical device under the skin, creates no chest scarring, and requires no lead or lead wire, all of which can mean fewer post-implant activity restrictions. In a clinical trial, the Micra reduced complications by 51 percent compared with traditional pacemakers.
contraction timing. Medtronics’ Micra pacemaker device eliminates these wire leads, which can sometimes malfunction or cause infections in surrounding tissues, necessitating a surgical procedure to replace the device. Due to Micra’s miniature size and minimally invasive installation approach, it leaves no lump or other visible sign of a medical device under the skin, creates no chest scarring, and requires no lead or lead wire, all of which can mean fewer post-implant activity restrictions. In a clinical trial, the Micra reduced complications by 51 percent compared with traditional pacemakers.
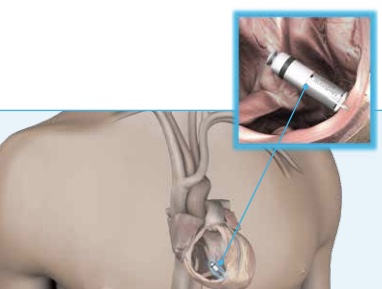 The Micra TPS is implanted directly into the heart via the femoral vein (in the leg) using a catheter delivery system, and attached to the right ventricle with four small prongs (tines), which contain steroids to reduce inflammation at the implantation site.
The Micra TPS is implanted directly into the heart via the femoral vein (in the leg) using a catheter delivery system, and attached to the right ventricle with four small prongs (tines), which contain steroids to reduce inflammation at the implantation site.  After the pacemaker is implanted, a physician tests and programs the device. The Micra’s cardiocapsule is completely self-contained within the heart and provides the therapy needed without a visible reminder that the patient is wearing a medical device.
After the pacemaker is implanted, a physician tests and programs the device. The Micra’s cardiocapsule is completely self-contained within the heart and provides the therapy needed without a visible reminder that the patient is wearing a medical device.
The FDA says the Micra TPS is indicated for use in patients who have slow or irregular heart rhythms and who may benefit from a single chamber pacemaker system or when placement of a traditional system is difficult.
The regulatory agency reports that it evaluated data from a clinical trial of 719 patients implanted with the Micra TPS, finding that in this study, 98 percent of patients had adequate heart pacing (known as pacing capture threshold) six months after the device was implanted. Complications occurred in fewer than 7 percent of participants in the clinical trials, and included prolonged hospitalizations, blood clots in the legs (deep vein thrombosis) and lungs (pulmonary embolism), heart injury, device dislocation, and heart attacks.
 “As the first leadless pacemaker, Micra offers a new option for patients considering a single chamber pacemaker device, which may help prevent problems associated with the wired leads,” said William Maisel, M.D., MPH, acting director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, in a press release. “Micra is intended for patients with a heart arrhythmia called atrial fibrillation or those who have other dangerous arrhythmias, such as bradycardia-tachycardia syndrome.”
“As the first leadless pacemaker, Micra offers a new option for patients considering a single chamber pacemaker device, which may help prevent problems associated with the wired leads,” said William Maisel, M.D., MPH, acting director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, in a press release. “Micra is intended for patients with a heart arrhythmia called atrial fibrillation or those who have other dangerous arrhythmias, such as bradycardia-tachycardia syndrome.”
An ongoing clinical trial called the Micra Transcatheter Pacing Study is continuing to evaluate the safety and efficacy of the Micra Transcatheter Pacing System and to assess long-term performance. The trial is expected to be conducted in up to 70 sites around the world, including up to 35 sites in the United States. Participating areas may include Europe, China, India, Malaysia, and Japan. Up to 780 subjects will be enrolled to allow up to 720 subjects to be implanted, enabling at least 600 subjects to be followed for at least 12-months post-implant.
All subjects will be followed until the official end of study (official study closure is defined as when Medtronic and/or FDA requirements have been satisfied per the Clinical Investigational Plan and/or by a decision by Medtronic or regulatory authority).
Additionally, the Micra Accelerometer Sensor Sub-Study (MASS) is expected to be conducted at four centers already active in the Micra study, and is intended to include countries such as Austria, Spain, Hungary and France. Up to 40 subjects are expected to be enrolled in the sub-study. The purpose of the sub-study is to test feasibility for future enhancements in the functionality of the Micra device.
For more information about the Micra Transcatheter Pacing System, a brochure is available here.



