Danvers, Massachusetts based in Abiomed, Inc., specialists in heart support technologies and circulatory boosting medical devices, has received U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA) for its Impella 2.5 System, a miniature blood pump system designed and engineered to maintain stable heart function and circulation during certain elective and urgent high-risk percutaneous coronary intervention (HRPCI) procedures.
The FDA’s approval is based on extensive clinical data submitted by Abiomed supporting this PMA as part of the 515 initiative. Under amendments to Section 515 of the FD&C Act made in 2012, the FDA must issue proposed and final orders to call for PMAs for 515 devices or reclassify them by holding a device classification panel meeting to consider the classification of these devices.
Abiomed’s Impella 2.5 is the first hemodynamic support device to receive a PMA indication for use certain high-risk percutaneous coronary intervention (HRPCI) procedures, including balloon angioplasty and stenting, which re-open coronary arteries that are narrowed or blocked as a consequence of severe coronary artery disease (CAD), demonstrating the device’s safety and effectiveness for this complex patient category.
Coronary artery disease can lead to chest pain and heart attack and is the leading cause of death for both men and women in the U.S. CAD occurs when one or more major arteries on the heart’s surface becomes narrow or blocked, reducing blood flow and starving the heart muscle of oxygen-rich blood.
With the FDA approval, the Impella 2.5 System is indicated for temporary ( 6 hours) ventricular support during high risk PCI performed in elective or urgent hemodynamically stable patients with severe symptomatic CAD and depressed left ventricular ejection fraction are undergoing HRPCI but who are not candidates for surgical coronary bypass treatment. In patients with diminished heart function, the heart pumps less blood than normal every time it beats, a condition sometimes associated with CAD.
Per the 2011 ACC/AHA guidelines, Impella 2.5 is a Class 1 recommendation for a heart team including a cardiac surgeon, to determine whether a treatment strategy for revascularization (either high risk PCI or surgery) is an appropriate therapeutic alternative. This decision is made based either on a pre-defined institutional protocol or on a per patient basis. The heart team approach has also been employed in determining treatment strategy for heart valve replacement.
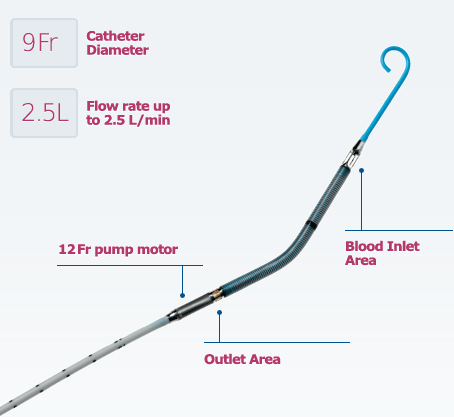
If the protocol determines that PCI is appropriate, Impella is the only hemodynamic support device proven safe and effective for high risk PCI. The Impella 2.5 helps maintain stable heart function during surgery by drawing blood from the left lower chamber of the heart (left ventricle) and pumping it into the aorta, the main blood vessel leading away from the heart that supplies oxygen-rich blood to the body. Use of the device in these patients may prevent hemodynamic instability that can occur during planned temporary coronary occlusions and may reduce peri- and post-procedural adverse events.
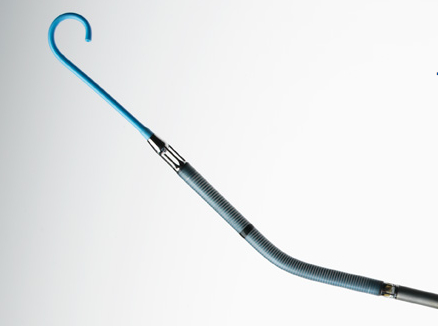
Prior to beginning a procedure, the cardiologist places the Impella 2.5 using a catheter with the pump loaded into its tip. The catheter is inserted into one of the body’s large arteries — usually in the leg, then guided through the patient’s arteries and into the left ventricle. Once the device is in place, an external controller and monitor turns the pump on and off, measures heart function, and allows the surgical team to adjust pumping as required in order to maintain stable heart function and circulation during the procedure.
The product labeling also authorizes the surgeon to make a clinical decision to leave Impella 2.5 in place beyond the intended 6 hour duration in instances of unforeseen circumstances.
Any patient undergoing HRPCI is by definition at some risk for complications related to decreased heart function and lowered blood pressure during the procedure. However, patients needing treatment for extensive or critically located CAD who are already experiencing diminished heart function are already at high risk. Unstable heart function during performance of an HRPCI procedure can result in serious complications or even prevent completion of the procedure. “Use of the Impella 2.5 System is intended to prevent episodes of unstable heart function, including unstable blood pressure and poor circulation, in patients who are at high risk for its occurrence,” explains William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health.
“The rigorous data from FDA clinical trials such as PROTECT I and PROTECT II demonstrate that complex, high-risk patients undergoing protected PCI with Impella 2.5 support experience reduced adverse events, improved quality of life and are able to return home faster with fewer repeat procedures,” says William O’Neill, M.D. of the Henry Ford Hospital in Detroit. “The heart team approach has evolved into a mainstream practice recognized by the guidelines for determining the need for PCI versus surgery and will continue to act as a platform for the screening and determination of the appropriate revascularization treatment for this high risk patient population.”
The FDA reviewed data pertaining to the Impella 2.5 System in a premarket approval application, which is the agency’s standard protocol for evaluating a reasonable assurance of safety and effectiveness for class III medical devices. Data supporting approval of the Impella 2.5 System included clinical results from the Abiomed’s PROTECT II clinical study with supporting information obtained from the USpella Registry, a multi-center, observational registry.
In addition to the U.S. clinical trial data, Abiomed’s Impella 2.5 PMA submission included clinical and scientific supporting evidence from more than 215 publications, totaling 1,638 Impella 2.5 patients and incorporated a medical device reporting (MDR) analysis from 13,981 Impella 2.5 patients. In addition to PROTECT I and PROTECT II, the submission included further data from 637 high risk patients enrolled in the U.S. Impella registry — an ongoing multicenter, observational retrospective registry including 49 centers. Data collection from the registry includes Institutional Review Board (IRB) approval, complete data monitoring and Clinical Events Committee adjudication. The PMA analysis also included hemodynamic science described in the literature that was validated by a series of pre-clinical and clinical studies.
The FDA notes that the overall data submitted provided evidence that for patients with severe CAD and diminished heart function, temporary circulatory support provided by Abiomed’s Impella 2.5 System during a HRPCI procedure may allow for longer and more thorough procedure, by preventing episodes of hemodynamic instability (e.g., poor circulation, low blood pressure) due to temporary heart function abnormalities in. Moreover, the agency determined that fewer subsequent adverse events (e.g., need for repeat HRPCI procedures) may occur in patients undergoing HRCPI with the pump compared to patients undergoing HRPCI with an intra-aortic balloon pump (IABP), and that the Impella 2.5 System can be used as an alternative to the IABP without significantly increasing the safety risks of the HRPCI procedure.
 “Abiomed would like to thank the FDA and all the dedicated caregivers and investigators for this achievement. The FDA approval of the Impella 2.5 device is one of our most significant milestones, representing a clinical advancement for physicians and patients. The FDA’s recognition of this elective and urgent patient population is an important acknowledgement of their growing need for treatment. As heart disease patients get sicker, more complex, and desire minimally invasive solutions, there are few options available to them to help improve their quality of life in a cost effective manner,” says Abiomed Chairman, President and Chief Executive Officer Michael R. Minogue.”We are excited that Impella has been recognized as a device that can potentially become the new standard of care with a ‘first of its kind’ approval.”
“Abiomed would like to thank the FDA and all the dedicated caregivers and investigators for this achievement. The FDA approval of the Impella 2.5 device is one of our most significant milestones, representing a clinical advancement for physicians and patients. The FDA’s recognition of this elective and urgent patient population is an important acknowledgement of their growing need for treatment. As heart disease patients get sicker, more complex, and desire minimally invasive solutions, there are few options available to them to help improve their quality of life in a cost effective manner,” says Abiomed Chairman, President and Chief Executive Officer Michael R. Minogue.”We are excited that Impella has been recognized as a device that can potentially become the new standard of care with a ‘first of its kind’ approval.”
The Impella 2.5, which received 510(k) clearance from the FDA in 2008, is supported by clinical guidelines, and its use has been reimbursed by the Centers for Medicare & Medicaid Services (CMS) under ICD-9-CM code 37.68 since 2008 for multiple indications, including high risk PCI. Per requirements of the FDA regulatory process, Abiomed will conduct a single arm, post-approval study on the Impella 2.5, collecting data on high risk PCI patients.
For more information about the Impella 2.5 system, visit: http://www.abiomed.com/?gclid=CNO7v9L20sQCFQ8oaQodjZcAHA
Sources:
The U.S. Food and Drug Administration (FDA)
Abiomed, Inc.
Image Credits:
Abiomed, Inc.