The research team behind Houston-based Procyrion, Inc.’s Aortix long-term circulatory support pump for chronic heart failure patients will deliver a presentation at the 2015 International Society for Heart and Lung Transplantation (ISHLT) 35th Annual Meeting & Scientific Session in Nice, France.
The Procyrion scientists will address the question of whether a long-term power lead exiting the femoral artery is feasible for powering the company’s marquee product, the Aortix long-term intravascular circulatory support pump for chronic heart failure patients, and describe their methods of study, in a presentation at the conference on Wednesday, April 15, 2015.
In conference abstract presentation 469, presenting author Dr. Reynolds M. Delgado III, Procyrion’s Founder and Chief Medical Officer, and company co-authors will relate their pre-clinical experience with a transfemoral power lead for Aortix, and report their findings during 30 days of observation following percutaneous deployment of the device in large animal studies, in which subjects showed no bleeding complications, ambulated normally, and were free from infection, swelling, and hemotoma.
 Dr. Delgado is a practicing cardiologist and a research leader in the field of heart failure, and a long-standing research collaborator with NASA Johnson Space Center’s human space flight physiology laboratory. He was an author on two NEJM articles that led to FDA approval of the Heartmate II LVAD, Principal Investigator on the ORQIS MOMENTUM Trial, Basic Science Researcher in Heart Failure, THI, Baylor College of Medicine and UT Houston Medical School, and Co-founder and President of the Houston Heart Failure Society, Dr. Delgado created the heart failure clinic program at Baylor St. Luke’s Medical Center, and is on the Transplant Medical Review Board there. As a result of his extensive experience in the heart failure and transplant arenas, he was one of 43 physicians worldwide asked to develop the Guidelines for the Care of Heart Transplant Recipients by the International Society for Heart and Lung Transplantation. He also authored Mechanical Circulatory Support in Patients with Heart Failure, included in the definitive textbook, Heart Failure: A Companion to Braunwald’s Heart Disease
Dr. Delgado is a practicing cardiologist and a research leader in the field of heart failure, and a long-standing research collaborator with NASA Johnson Space Center’s human space flight physiology laboratory. He was an author on two NEJM articles that led to FDA approval of the Heartmate II LVAD, Principal Investigator on the ORQIS MOMENTUM Trial, Basic Science Researcher in Heart Failure, THI, Baylor College of Medicine and UT Houston Medical School, and Co-founder and President of the Houston Heart Failure Society, Dr. Delgado created the heart failure clinic program at Baylor St. Luke’s Medical Center, and is on the Transplant Medical Review Board there. As a result of his extensive experience in the heart failure and transplant arenas, he was one of 43 physicians worldwide asked to develop the Guidelines for the Care of Heart Transplant Recipients by the International Society for Heart and Lung Transplantation. He also authored Mechanical Circulatory Support in Patients with Heart Failure, included in the definitive textbook, Heart Failure: A Companion to Braunwald’s Heart Disease
 “The challenges to this type of mechanical circulatory support implantation include mitigating driveline infection and bleeding risk. While we continue to investigate the safety of this technique, these positive results help us shift focus to other critical areas required to advance this technology,” says Jason Heuring, Ph.D., Procyrion’s Chief Operating Officer, in a release.
“The challenges to this type of mechanical circulatory support implantation include mitigating driveline infection and bleeding risk. While we continue to investigate the safety of this technique, these positive results help us shift focus to other critical areas required to advance this technology,” says Jason Heuring, Ph.D., Procyrion’s Chief Operating Officer, in a release.
Procyrion’s presentation abstract addressing its novel work on Aortix has been vetted and accepted by a ISHLT Abstract Selection Committee whose members include scientists, infectious disease specialists, tissue engineers, and leading surgeons from around the world.
The International Society for Heart and Lung Transplantation is a multidisciplinary, professional organization dedicated to improving care of patients with advanced heart or lung disease through transplantation, mechanical support, and innovative therapies via research, education, and advocacy.
There is also a ISHLT 2015 Mobile Meeting App for iOS and Android devices available. A FAQ on the app is available here.
In June, the Procyrion team is also slated to attend the 2015 Gordon Research Conference (GRC) on Assisted Circulation in Lucca, Italy.
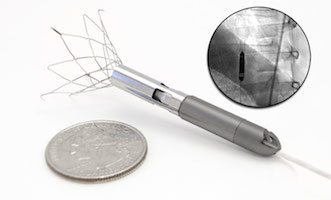
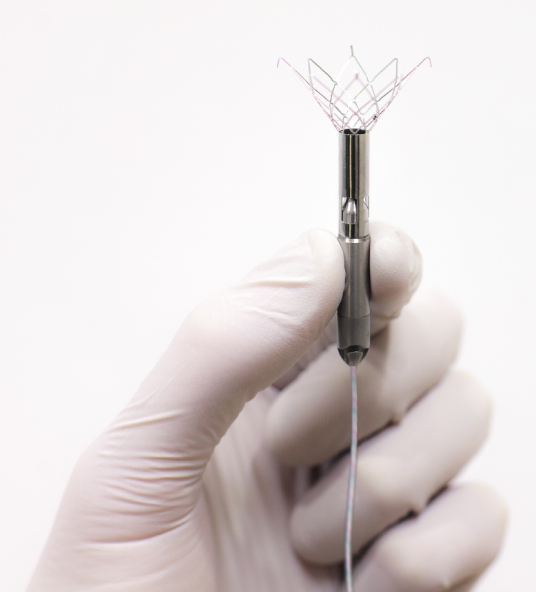
Conceived by Dr. Delgado, Aortix is expected to provide another treatment option for millions of chronic heart failure patients too sick for medication alone to be effective. The device, thinner than a #2 pencil, consists of a small, continuous flow pump mounted within a self-expanding anchoring system.
The device is delivered via a catheter inserted through the femoral artery to the descending thoracic aorta. Once in place, self-expanding anchors deploy to fix the pump to the aortic wall. Following deployment, the catheter is fully removed, leaving only the anchored pump and a small diameter, flexible electrical power wire which may be tunneled to a desired transdermal exit site or to a Transcutaneous Energy Transfer (TET) system for subcutaneous implantation without an indwelling power lead. Procyrion says that compared to traditional LVADs, its device’s small size and unique design enable placement to be as quick and simple as a ten-minute outpatient procedure, replacing major surgery and a lengthy hospital stay with a minimally invasive outpatient procedure.
Giving The Heart An Opportunity To Rest And Heal
The Procyrion device is intended to reduce afterload, reduce workload of the heart, and increase end-organ perfusion. Together, these factors provide potential to rest and heal the heart thereby improving patient quality of life and reducing the cost of heart failure.
 Research on the device conducted by QTest labs in sheep with ischemic chronic heart failure demonstrated that Aortix reduces heart workload and decreases oxygen demand, which may help improve heart rest and healing while dramatically improving kidney function. “These results exceeded our expectations and is further evidence that Aortix could be used to support the failing heart before years of progressive damage occur,” comments Procyrion Director of Research and Development, Will Clifton, MD, in a release.
Research on the device conducted by QTest labs in sheep with ischemic chronic heart failure demonstrated that Aortix reduces heart workload and decreases oxygen demand, which may help improve heart rest and healing while dramatically improving kidney function. “These results exceeded our expectations and is further evidence that Aortix could be used to support the failing heart before years of progressive damage occur,” comments Procyrion Director of Research and Development, Will Clifton, MD, in a release.
The Procyrion device reduces risk in three main areas:
Minimal Procedural Risk
Procyrion is currently developing a minimally invasive, cardiologist friendly, catheter-lab tool and developing an outpatient procedure that it says will dramatically reduce procedural risks as compared to full support circulatory assist devices that require invasive surgery and 40 day hospital stays on average. Unlike other circulatory assist devices, the Procyrion device is deployed downstream of the heart, eliminating the risk of damaging the heart or its valves, and simplifying deployment.
Minimal Thrombosis Risk
Procyrion observes that the most severe risk for patients with circulatory support devices is thrombotic stroke, with up to 40 percent of patients with surgical ventricular assist devices ending up with a stroke. Moreover, many of these patients will be on a heart transplant wait lists, and, subsequent to a stroke will no longer be transplant eligible. As noted, because the Procyrion device is deployed downstream of the carotid arteries that supply the brain it poses minimal thrombotic stroke risk. Downstream thrombotic events are rendered more unlikely since the Procyrion device functions in series with the heart, without disrupting native pulsatile flow. This minimizes stasis, since the device is continuously flushed with high volumes of native blood flow.
Minimal Failure Risk
Traditional support devices replace the heart’s function as opposed to supporting native heart function, and mean that a pump failure will nearly always be fatal. Contrarily, in the unlikely event of Procyrion pump failure, the device’s small diameter will not obstruct native blood flow and therefore not result in a life threatening event. Additionally, if the pump fails, the system can be easily retrieved and replaced using standard catheter based procedures.
Novel pumping paradigm
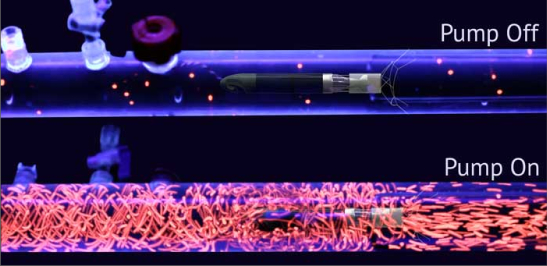
Traditional circulatory assist devices are deployed across a valve or bypass entire segments of the native circulation by plumbing one inch holes in the heart and aorta. Conversely, Procyrion’s intra-aortic pump utilizes a small diameter axial flow pump and fluid entrainment (aka “jet pumping”) in order to entrain and efficiently transfer energy to the native aortic flow. The device supports the natural function of the heart instead of fully replacing it.
Procyrion’s intra-aortic pump utilizes fluid entrainment to augment native blood flow resulting an acceleration of fluid, a net increase in cardiac output, and reduced workload reduction for the heart through afterload reduction. The company says this partial support system is well-suited to patients in NYHA Stage III – early Stage IV heart failure who don’t need full cardiac replacement. In addition, the device’s novel fluid entrainment approach enables an extremely low profile design, rendering it suitable for minimally invasive, trans-catheter deployment and retrieval. Procyrion also notes that fluid entrainment has been used extensively in mining, refrigeration, in-line mixing, and other industrial applications to promote efficient mixing and flow.
Sources:
Procyrion Inc.
QTest Laboratories
International Society for Heart and Lung Transplantation (ISHLT)
Texas Heart Institute
Baylor St. Luke’s Medical Center
The American College of Cardiology
The International Society for Heart and Lung Transplantation
Image Credits:
Procyrion Inc.
QTest Laboratories