In a recent study entitled “Advanced Age Protects Microvascular Endothelium from Aberrant Ca2+ Influx and Cell Death Induced by Hydrogen Peroxide” a research team at the University of Missouri shows that advanced age can actually induce mechanisms to protect endothelium from injury. The study was published in the Physiological Society’s Journal of Physiology.
Aging is the underlying cause of many diseases, many of which the triggering mechanism is still unknown. However, it is well established that oxidative stress is a leading cause for many aging-related diseases, such as the common diabetes, hypertension and several types of cancer, as Steven Segal, PhD, a professor of medical pharmacology and physiology at the MU School of Medicine and study lead author explained, “Molecules known as reactive oxygen species, or ROS, play an important role in regulating cellular function. However, the overproduction of ROS can help create a condition referred to as oxidative stress, which can alter the function of cells and interfere with their growth and reproduction.”
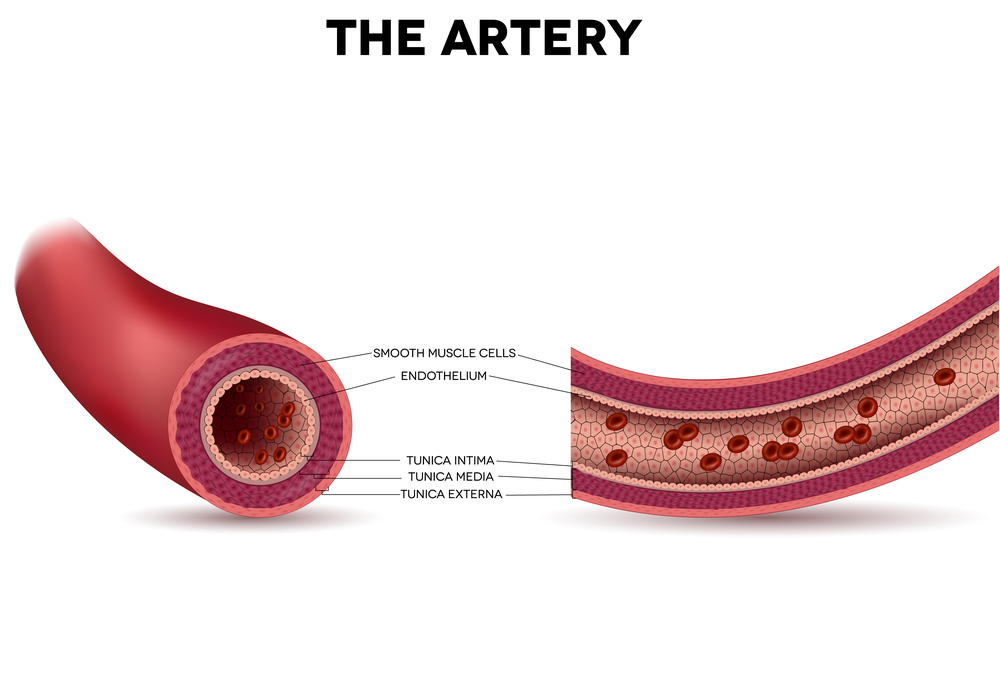
In this new study, researchers at the MU School of Medicine show that age can actually protect against oxidative stress. The team focused on the effects of aging on blood vessels endothelium (i.e., the tissue layer lining the interior surface of blood and lymphatic vessels) when exposed to oxidative stress. They studied these effects in small arteries, since endothelium integrity is crucial for arteries resistance, a key condition for cardiovascular function.
Dr. Segal explained the team’s experimental setup, “We studied the endothelium from resistance arteries of male mice at 4 months and 24 months of age, which correspond to humans in their early 20s and mid-60s. We first studied the endothelium under resting conditions and in the absence of oxidative stress. We then simulated oxidative stress by adding hydrogen peroxide. When oxidative stress was induced for 20 minutes, the endothelial cells of the younger mice had abnormal increases in calcium when compared to the endothelial cells of the older mice. This finding is important because when calcium gets too high, cells can be severely damaged.”
Pushing the system to its limit, the scientists prolonged oxidative stress for 60 minutes. They observed that young mice presented a significant increase in endothelium cells death when compared to older mice, specifically 7-fold increase. Thus, the teams’ findings suggest that endothelium of aged mice can trigger mechanisms that protect it from oxidative-induced damage.
This phenotype is against the paradigm that age only enhances damage, as Dr. Segal noted, “The most surprising thing we found is that the endothelium was much less perturbed by oxidative stress during advanced age when compared to younger age. This finding contrasts with the generally held belief that the functional integrity of the endothelium is compromised as we age. Our study suggests that blood vessels adapt during the aging process to regulate ROS and minimize cell death when subjected to an abrupt increase in oxidative stress. This adaptation helps to ensure that the arteries of older individuals can still do their jobs.”
These findings advance our understanding of aging-related diseases and suggest that some mechanisms can actually protect and promote tissues healthy-function.
As Dr. Segal concluded, “Although more studies are needed to identify the mechanism by which the endothelium adapts to advanced age, our study provides evidence that the natural tendency of the body is to adapt to oxidative stress during healthy aging.”
[adrotate banner=”25″]